Why green hydrogen ?
Green hydrogen: its extraordinary power
to substitute to fossil fuels
Producing green hydrogen
Hydrogen is only available in infinitesimal quantities in pure form in nature. It can be produced from fossil fuels (steam methane reforming) in a process which emits large quantities of CO2. Hydrogen can also be produced by electrolysis from water, a process invented by two British chemists in 1800, in a reaction that also produces oxygen: 2 H2O + electricity = 2 H2 + O2 + heat. The electrical activation between a cathode and an anode creates a chemical reaction. The energy content of water is considerable: 1 liter of water contains the equivalent of 0.4 liter of oil.
Electrolysis was industrially developed in the early 20th century and is now being ramped up in electrolyzer gigafactories, bringing about a virtuous circle of higher volumes and lower costs. When produced from solar power, hydrogen (which is then called green) is a virtually infinite, zero-carbon commodity.

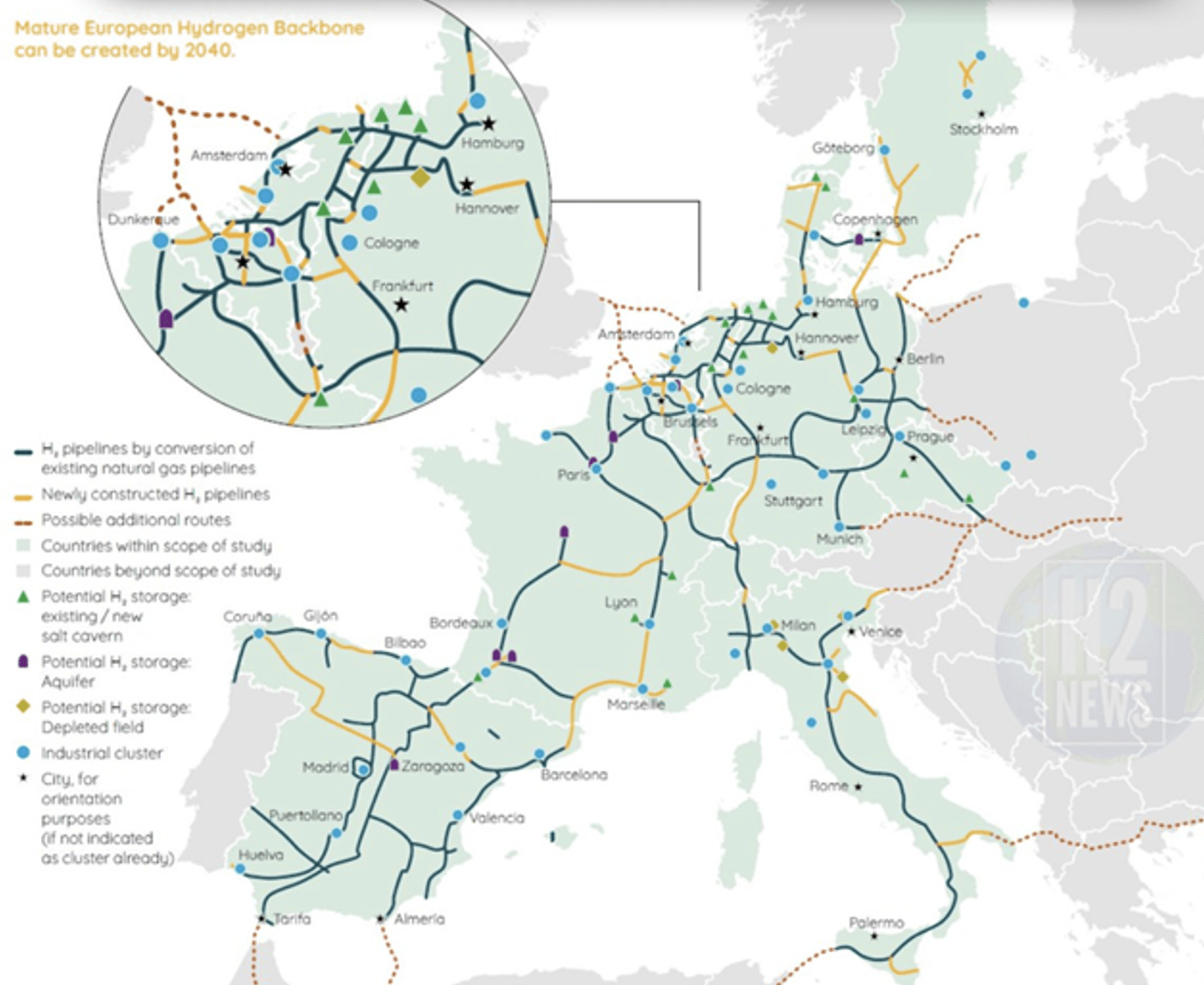
Securing hydrogen delivery
Hydrogen has been safely transmitted over pipelines for decades. Repurposing natural gas grids is the fastest route to bring hydrogen from mass production areas in the high-resource areas in the Mediterranean region to urban and industrial hubs all over Europe.
Mass-scale underground storage in salt caverns and aquifers offers a potential of hundreds of TWh (over 10,000 times the global electron storage capacity in batteries), ensuring secure and timely delivery, night and day throughout the year.
Universal applications
Hydrogen can replace coal, oil and natural gas in virtually all applications, turning heavily polluting and CO2 emitting industries into clean and zero-carbon ones.
Heat and CHP Generation
Convert oil, gas, coal fired CHP and district heating installations
Baseload Power Generation
Convert coal and gas fired plants into hydrogen-based power plants
Green Ammonia Production
Convert traditional ammonia plants into green plants using hydrogen from renewable sources
Green Steel Production
Convert existing steel plants from coking coal to Direct Reduced Iron with hydrogen
Green Methanol Production
Use green hydrogen to take advantage of the CO2 emissions and produce zero carbon methanol
Fuel-Cell Trucks and Trains
Substitute fuel cells for diesel in trucks and trains

